Label all bonds in ch2br2 . – Labeling bonds in CH2Br2 is a crucial step in understanding the molecular structure and properties of this compound. In this comprehensive guide, we will delve into the types of bonds present in CH2Br2, their polarity, and their impact on the overall molecular geometry and physical properties.
CH2Br2, also known as methylene bromide, is a versatile organic compound with applications in various fields. By understanding the bonding characteristics of CH2Br2, we can gain insights into its reactivity and behavior in different chemical reactions.
Covalent Bonds in CH2Br2
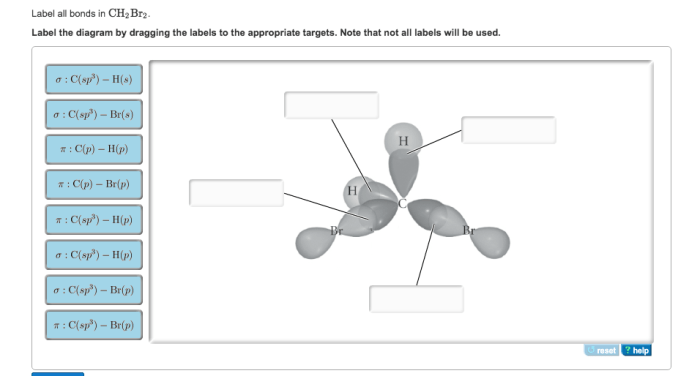
Covalent bonding is a type of chemical bond that involves the sharing of electron pairs between atoms. In a covalent bond, the electrons are not transferred from one atom to another, but rather are shared between the two atoms.
In CH2Br2, each carbon atom is bonded to two hydrogen atoms and two bromine atoms by covalent bonds. The carbon-hydrogen bonds are formed by the sharing of one pair of electrons between the carbon atom and each hydrogen atom. The carbon-bromine bonds are formed by the sharing of one pair of electrons between the carbon atom and each bromine atom.
Polar Covalent Bonds
The covalent bonds in CH2Br2 are polar covalent bonds. This means that the electrons are not shared equally between the atoms. The bromine atoms are more electronegative than the carbon and hydrogen atoms, so they attract the electrons more strongly.
This results in a partial negative charge on the bromine atoms and a partial positive charge on the carbon and hydrogen atoms.
Polarity of Bonds in CH2Br2
Bond polarity refers to the unequal distribution of electrons in a covalent bond. In a polar bond, one atom has a greater share of the electrons than the other. This results in a partial positive charge on one atom and a partial negative charge on the other.
In CH2Br2, the bonds between carbon and hydrogen are nonpolar, while the bonds between carbon and bromine are polar. This is because bromine is more electronegative than carbon, meaning it has a greater attraction for electrons. As a result, the electrons in the C-Br bonds are pulled towards the bromine atoms, creating a partial negative charge on the bromine atoms and a partial positive charge on the carbon atom.
Electronegativity Difference
The electronegativity difference between two atoms is a measure of the tendency of one atom to attract electrons from the other. The greater the electronegativity difference, the more polar the bond will be.
In CH2Br2, the electronegativity difference between carbon and hydrogen is 0.4, while the electronegativity difference between carbon and bromine is 1.0. This means that the C-Br bonds are more polar than the C-H bonds.
Types of Bonds in CH2Br2
CH2Br2, also known as methylene bromide, is an organic compound composed of carbon, hydrogen, and bromine atoms. It contains various types of chemical bonds that hold these atoms together.
Covalent Bonds
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In CH2Br2, the carbon atom shares two electrons with each of the two hydrogen atoms, forming two C-H covalent bonds. The carbon atom also shares two electrons with each of the two bromine atoms, forming two C-Br covalent bonds.
These covalent bonds are polar due to the difference in electronegativity between carbon and hydrogen/bromine. The carbon-bromine bonds are more polar than the carbon-hydrogen bonds because bromine is more electronegative than hydrogen.
Polar Covalent Bonds
Polar covalent bonds are covalent bonds in which the electrons are not shared equally between the atoms. In CH2Br2, the C-Br bonds are polar covalent bonds because the bromine atom is more electronegative than the carbon atom. This means that the electrons in the C-Br bonds are pulled more towards the bromine atom, creating a partial negative charge on the bromine atom and a partial positive charge on the carbon atom.
Nonpolar Covalent Bonds
Nonpolar covalent bonds are covalent bonds in which the electrons are shared equally between the atoms. In CH2Br2, the C-H bonds are nonpolar covalent bonds because the carbon atom and the hydrogen atom have similar electronegativities. This means that the electrons in the C-H bonds are shared equally between the carbon and hydrogen atoms, creating no partial charges.
Bond Lengths and Bond Strengths in CH2Br2
Bond length refers to the distance between the nuclei of two bonded atoms, while bond strength measures the energy required to break a bond. In CH2Br2, there are two types of bonds: C-H bonds and C-Br bonds.
C-H Bond Length and Strength
The C-H bond length in CH2Br2 is approximately 1.10 Å (angstroms), and its bond strength is around 414 kJ/mol (kilojoules per mole). The relatively short bond length and high bond strength indicate a strong bond between the carbon and hydrogen atoms.
Labeling all the bonds in CH2Br2 can be a bit tricky, but it’s important to understand the different types of bonds present. For example, the C-H bonds are polar covalent bonds, while the C-Br bonds are nonpolar covalent bonds. This difference in bond polarity is due to the difference in electronegativity between carbon and hydrogen, and carbon and bromine.
In other news, joel earns 1700 per month . Continuing on with our discussion of CH2Br2, the molecular geometry of the molecule is tetrahedral, with the carbon atom at the center of the tetrahedron. The two hydrogen atoms and two bromine atoms are located at the corners of the tetrahedron.
C-Br Bond Length and Strength, Label all bonds in ch2br2 .
The C-Br bond length in CH2Br2 is approximately 1.94 Å, which is longer than the C-H bond. The C-Br bond strength is also lower, at around 276 kJ/mol. The longer bond length and lower bond strength suggest a weaker bond between the carbon and bromine atoms compared to the C-H bond.
Factors Affecting Bond Length and Strength
Several factors influence bond length and strength, including:
-
-*Atomic size
Larger atoms generally form longer bonds.
-*Electronegativity
The difference in electronegativity between two atoms affects bond polarity and strength.
-*Bond order
Bonds with higher bond orders (e.g., double or triple bonds) are shorter and stronger.
-*Hybridization
The hybridization of the atomic orbitals involved in bonding can influence bond length and strength.
In CH2Br2, the larger size of bromine compared to hydrogen contributes to the longer C-Br bond length. Additionally, the difference in electronegativity between carbon and bromine (2.55 and 2.96, respectively) results in a polar C-Br bond, weakening its strength compared to the C-H bond.
Resonance in CH2Br2
Resonance is a concept in chemistry that describes the delocalization of electrons within a molecule. It occurs when a molecule can be represented by multiple Lewis structures that have the same number of electrons but different arrangements of double and single bonds.In
the case of CH2Br2, resonance occurs because the double bond between the carbon and one of the bromine atoms can be represented in two different ways. In one resonance structure, the double bond is between the carbon and the bromine atom on the left, while in the other resonance structure, the double bond is between the carbon and the bromine atom on the right.The
two resonance structures of CH2Br2 are shown below:“`CH2=Br-Br <--> CH2-Br=Br“`The two resonance structures are equivalent in energy, and they contribute equally to the overall structure of the molecule. This means that the double bond in CH2Br2 is not localized to a single carbon-bromine bond, but rather it is delocalized over both carbon-bromine bonds.The delocalization of electrons in CH2Br2 makes the molecule more stable than it would be if the double bond were localized to a single carbon-bromine bond. This is because the delocalization of electrons allows the molecule to distribute its charge more evenly, which reduces the overall energy of the molecule.
Molecular Geometry of CH2Br2
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of valence electrons, the number of bonding pairs, and the number of lone pairs of electrons.
Molecular Geometry of CH2Br2
CH2Br2 has a tetrahedral molecular geometry. The carbon atom is at the center of the tetrahedron, with two hydrogen atoms and two bromine atoms bonded to it. The bond angles between the carbon atom and the hydrogen atoms are approximately 109.5 degrees, and the bond angles between the carbon atom and the bromine atoms are approximately 112 degrees.
Factors Determining Molecular Geometry
The following factors determine the molecular geometry of a molecule:
- The number of valence electrons
- The number of bonding pairs
- The number of lone pairs of electrons
Physical Properties of CH2Br2: Label All Bonds In Ch2br2 .
CH2Br2, also known as methylene bromide, exhibits a range of physical properties that are directly influenced by its molecular structure. The presence of two electronegative bromine atoms significantly impacts the molecule’s polarity, resulting in unique characteristics.
Physical Appearance
CH2Br2 is a colorless, volatile liquid at room temperature. It has a pungent, chloroform-like odor and is immiscible with water. The liquid’s density is 2.49 g/mL, and it has a boiling point of 97.6 °C and a melting point of -93.0 °C.
Polarity
The electronegative bromine atoms in CH2Br2 create a polar covalent bond with the carbon atom. This polarity results in a net dipole moment for the molecule, making it a polar molecule. The partial positive charge on the carbon atom and the partial negative charges on the bromine atoms contribute to the molecule’s polarity.
Solubility
CH2Br2 is immiscible with water due to its nonpolar nature. However, it is soluble in organic solvents such as chloroform, ether, and benzene. The nonpolar nature of the carbon-hydrogen bonds allows CH2Br2 to interact with other nonpolar molecules, leading to its solubility in organic solvents.
Toxicity
CH2Br2 is a toxic substance and should be handled with care. Inhalation of CH2Br2 vapors can cause irritation to the respiratory system, while skin contact can lead to dermatitis. It is also a suspected carcinogen, and prolonged exposure can cause damage to the liver and kidneys.
FAQ Explained
What is the hybridization of the carbon atom in CH2Br2?
The carbon atom in CH2Br2 is sp3 hybridized.
Why is CH2Br2 a polar molecule?
CH2Br2 is a polar molecule due to the electronegativity difference between carbon and bromine, resulting in a net dipole moment.
What is the molecular geometry of CH2Br2?
The molecular geometry of CH2Br2 is tetrahedral.