Decay series of uranium-238 answer key – Delve into the intricate world of the decay series of uranium-238, where alpha and beta particles dance in a mesmerizing symphony of nuclear transformations. This comprehensive answer key unveils the secrets of radioactive decay, unlocking the mysteries of ancient rocks and illuminating the potential hazards lurking within.
From its applications in radioactive dating to its implications for environmental health, the decay series of uranium-238 offers a captivating exploration of nuclear science.
Uranium-238 Decay Series: Decay Series Of Uranium-238 Answer Key
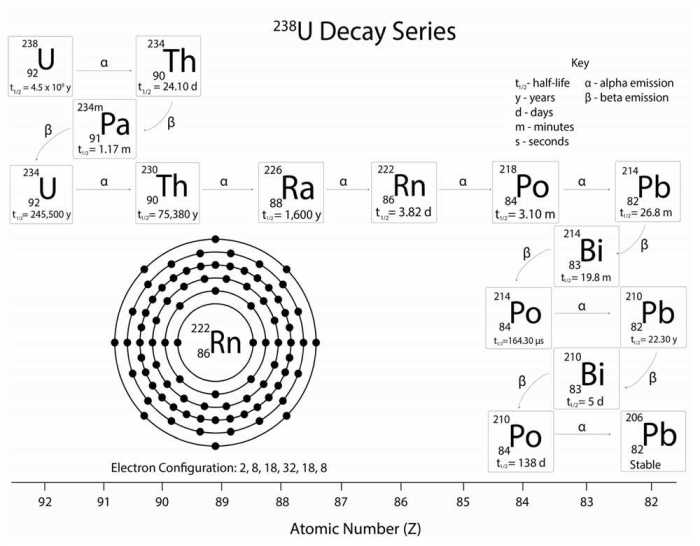
The uranium-238 decay series is a sequence of radioactive decays that begins with uranium-238 and ends with lead-206. The decay series includes a number of alpha and beta decays, and the half-lives of the isotopes involved range from a few milliseconds to billions of years.
Alpha and Beta Decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. Beta decay is a type of radioactive decay in which an atomic nucleus emits a beta particle, which is an electron or a positron.
In the uranium-238 decay series, alpha decay is the most common type of decay. Alpha particles are large and have a relatively low energy, so they cannot penetrate very far into matter. Beta particles are smaller and have a higher energy, so they can penetrate more deeply into matter.
Isotopes Involved in the Uranium-238 Decay Series, Decay series of uranium-238 answer key
The isotopes involved in the uranium-238 decay series, their half-lives, and the type of decay they undergo are summarized in the following table:
| Isotope | Half-life | Type of decay |
|---|---|---|
| Uranium-238 | 4.47 billion years | Alpha decay |
| Thorium-234 | 24.1 days | Beta decay |
| Protactinium-234 | 1.17 minutes | Beta decay |
| Uranium-234 | 245,500 years | Alpha decay |
| Thorium-230 | 75,380 years | Alpha decay |
| Radium-226 | 1,600 years | Alpha decay |
| Radon-222 | 3.8 days | Alpha decay |
| Polonium-218 | 3.05 minutes | Alpha decay |
| Lead-214 | 26.8 minutes | Beta decay |
| Bismuth-214 | 19.9 minutes | Beta decay |
| Polonium-214 | 164 microseconds | Alpha decay |
| Lead-210 | 22.3 years | Beta decay |
| Bismuth-210 | 5.01 days | Beta decay |
| Polonium-210 | 138.4 days | Alpha decay |
| Lead-206 | Stable | N/A |
Top FAQs
What is the half-life of uranium-238?
4.468 billion years
How is the decay series of uranium-238 used in radioactive dating?
By measuring the ratios of different isotopes in a sample, scientists can determine its age.
What are the potential environmental hazards associated with the decay of uranium-238?
Contamination of groundwater and soil, release of radioactive radon gas
How can exposure to uranium-238 and its decay products affect human health?
Increased risk of cancer, damage to DNA and cells