Rank the elements by effective nuclear charge – Delving into the realm of chemistry, the concept of effective nuclear charge (Zeff) emerges as a pivotal factor in understanding the chemical properties of elements. Zeff, a measure of the net positive charge experienced by electrons in an atom, plays a crucial role in shaping the periodic trends and reactivity of elements.
This exploration will delve into the methods for calculating Zeff, examining the influence of shielding effects and other factors. We will then embark on a journey through the periodic table, uncovering the periodic trends in Zeff and their impact on ionization energy, atomic radius, and chemical reactivity.
Finally, we will uncover the practical applications of Zeff in predicting and explaining chemical properties, highlighting its significance in fields such as catalysis and materials science.
1. Introduction: Rank The Elements By Effective Nuclear Charge

Effective nuclear charge (Z eff) is a fundamental concept in chemistry that describes the net positive charge experienced by an electron in an atom.
Z effplays a crucial role in understanding the chemical properties of elements and is a key factor in determining their behavior in chemical reactions.
2. Methods for Calculating Effective Nuclear Charge
Slater’s Rules, Rank the elements by effective nuclear charge
Slater’s rules provide a set of empirical equations to calculate Z efffor electrons in different orbitals.
These rules take into account the shielding effect, which is the reduction in the nuclear charge experienced by an electron due to the presence of other electrons in the atom.
Other Methods
Other methods for calculating Z effinclude the Hartree-Fock method and the density functional theory (DFT) method.
These methods are more accurate than Slater’s rules but require more computational resources.
3. Periodic Trends in Effective Nuclear Charge
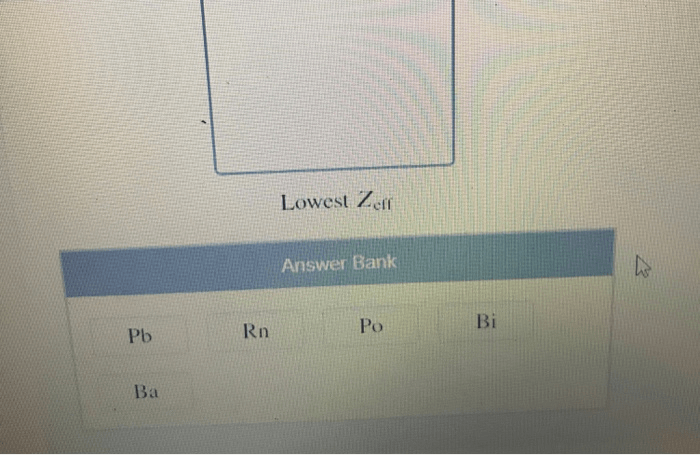
Z effincreases across a period from left to right due to the increase in atomic number.
Z effdecreases down a group due to the increase in the number of electron shells.
| Element | Z | Zeff |
|---|---|---|
| Li | 3 | 1.28 |
| Be | 4 | 1.91 |
| B | 5 | 2.42 |
| C | 6 | 3.14 |
| N | 7 | 3.83 |
4. Relationship between Effective Nuclear Charge and Chemical Properties
Ionization Energy
Z effis directly proportional to the ionization energy of an element.
The higher the Z eff, the more difficult it is to remove an electron from the atom.
Atomic Radius
Z effis inversely proportional to the atomic radius of an element.
The higher the Z eff, the smaller the atomic radius.
Chemical Reactivity
Z effinfluences the chemical reactivity of an element.
Elements with higher Z effare more likely to form ionic bonds, while elements with lower Z effare more likely to form covalent bonds.
5. Applications of Effective Nuclear Charge
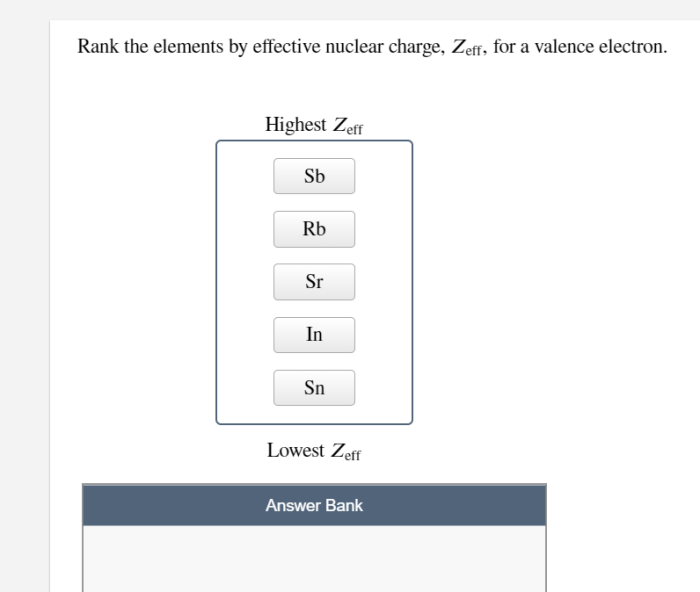
Z effis used to predict and explain a wide range of chemical properties.
It is used in catalysis to design catalysts with specific properties.
It is also used in materials science to understand the electronic structure of materials.
FAQ Resource
What is effective nuclear charge?
Effective nuclear charge (Zeff) is the net positive charge experienced by electrons in an atom, taking into account the shielding effect of inner electrons.
How is effective nuclear charge calculated?
Zeff can be calculated using Slater’s rules, which consider the number of electrons in each subshell and the shielding effect of inner electrons.
What is the significance of effective nuclear charge?
Zeff plays a crucial role in determining the chemical properties of elements, including ionization energy, atomic radius, and chemical reactivity.